David Rawn in Organic Chemistry Study Guide 2015 166 The Williamson Ether synthesis. The resonance stabilization in these two cases is very different.
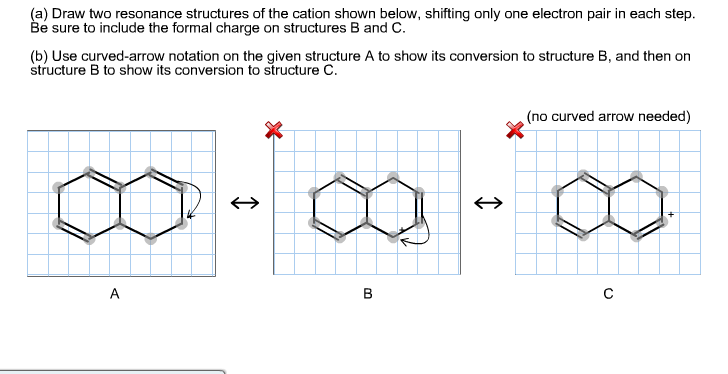
Solved Draw Two Resonance Structures Of The Cation Shown Chegg Com
However its measured structure is consistent with a description as a resonance hybrid of the two major contributing structures shown above.
. According to the contributing structures. HCl when benzoic acid gets. In the triethylamine and DABCO examples at the top of the table the cationic charge is relatively localized on a single nitrogen atom.
Looking more closely at the reaction we also noted two interesting patterns. The following table gives pK a values for some important nitrogen bases some structures for which are shown to its right. A similar set of resonance structures for the phenolate anion conjugate base appears below the phenol structures.
An important principle of resonance is that charge separation diminishes the importance of canonical contributors to the resonance hybrid and reduces the overall stabilization. Mass spectrometry MS is an analytical technique that is used to measure the mass-to-charge ratio of ionsThe results are presented as a mass spectrum a plot of intensity as a function of the mass-to-charge ratioMass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures. The filtrate is acidified with dil.
The nucleophile attacks at the more substituted position of the epoxide C-1 below inversion of stereochemistry occurs at this position but not at the other position note that the C-O bond at C-2 below is a wedge in both starting material and product. Academiaedu is a platform for academics to share research papers. Indeed since the chirality of the molecule preferentially enhances the intensity of the incident light with one spin.
Download eSaral App for Video Lectures Complete Revision Study Material and much more. The charge in lutidine may be delocalized onto ring carbons at the cost of aromatic stabilization. In summary it has been shown that hybrid systems composed of a nonchiral plasmonic nanoparticle and a chiral molecule behave distinctly with respect to the left- and right-circularly polarized incident light at the frequency of the plasmonic resonance.
It has two equal NO bonds of 125 pm intermediate in length between a typical NO single bond 145 pm in hydroxylamine H 2 NOH and NO double bond 115 pm in nitronium ion ONO. The last three examples are. Distillation of the solvent gives nitrobenzene.
The Williamson ether synthesis is an S N 2 reaction in which an alkoxide ion is a nucleophile that displaces a halide ion from an alkyl halide to give an ether. I The mixture is shaken with a dilute solution of mathrmNaHCO_3 and extracted with ether or chloroform when nitrobenzene goes into the organic layer. The reaction occurs with inversion of configuration at chiral centers and can be limited by possible competing.
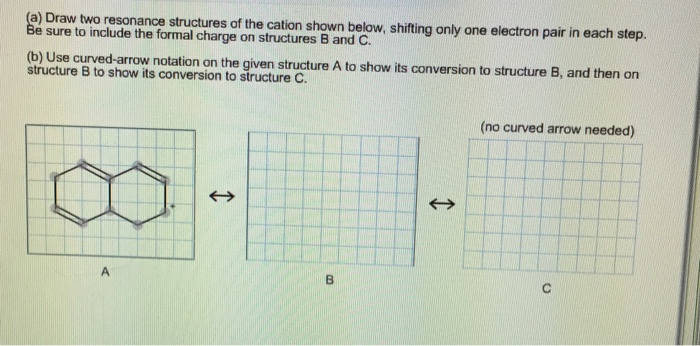
Solved Draw Two Resonance Structures Of The Cation Shown Chegg Com
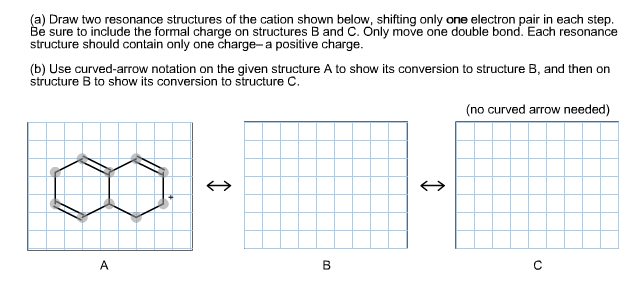
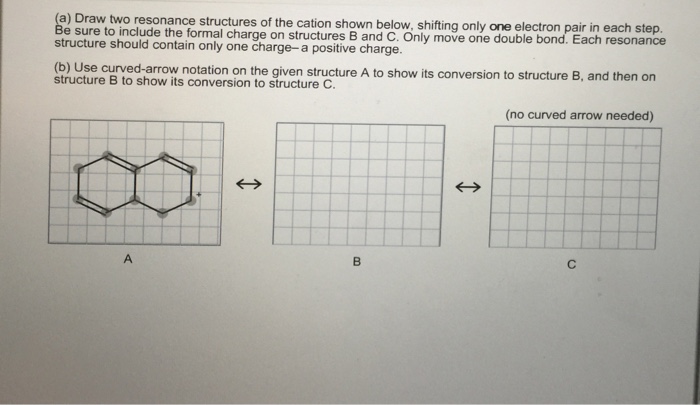
Solved A Draw Two Resonance Structures Of The Cation Shown Chegg Com
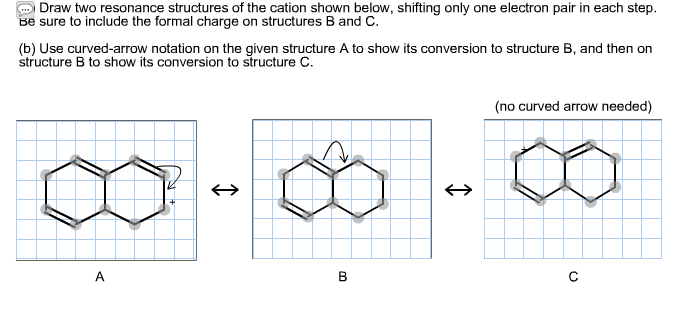
Solved Draw Two Resonance Structures Of The Cation Shown Chegg Com

Solved Draw Two Resonance Structures Of The Cation Shown Chegg Com
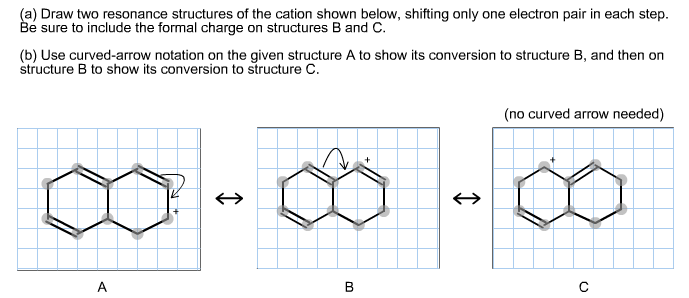
Solved Draw Two Resonance Structures Of The Cation Shown Chegg Com

Oneclass Draw Two Resonance Structures Of The Cation Shown Below Shifting Only One Electron Pair In

Get Answer A Draw Two Resonance Structures Of The Cation Shown Transtutors
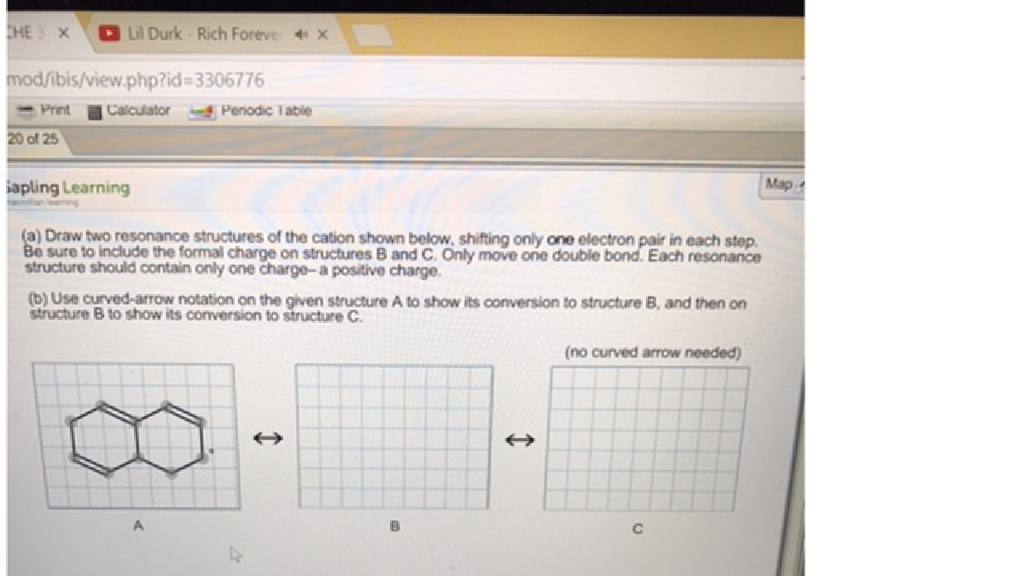
Solved Draw Two Resonance Structures Of The Cation Shown Chegg Com
0 comments
Post a Comment